2017-10-10
2016-11-08
WP5 is coordinated by Triantafyllos Chavakis (partner 5) in collaboration with partners 1, 7 and 9.
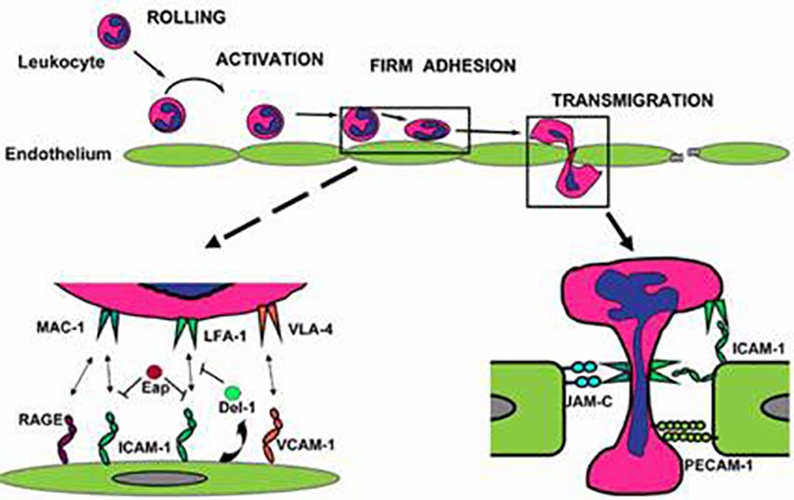
The objective of WP5 is to evaluate regulators targeted to intravascular immune responses by employing small-animal disease models and whole-blood assays.
The potency of the regulators as well as the blockade of the interaction between the platelet glycoprotein GPIb and the leukocyte integrin Mac-1 will be tested in whole-blood assays. More specifically, human or porcine endothelial cells, porcine islets or biomaterials will be incubated with blood in the presence of inhibitors. Innate responses will be examined by measuring the activation of cells, complement and coagulation. Del-1, which previously has been identified within WP5 as the first endogenous anti-adhesive molecule, will be tested in the preclinical models of kidney ischemia reperfusion injury and transplantation. The same will be done with some other immune inhibitors as well as the anti-inflammatory enzyme CD39.
In addition, the complement inhibitors will be evaluated in a model of Instant Blood-Mediated Inflammatory Reaction (IBMIR) that occurs after intraportal islet transplantation and involves most of the intravascular innate immune systems. To this point, diabetic mice will be transplanted with islets coated with the regulators and the achievement of normoglycemia will be assessed.
The accomplishment of the tasks within the WP5 will lead to the identification of molecules that can further be tested in WP6 in large animal models and also to the development of immune assays to evaluate murine models.